Jaime Andrés Lasso Pineda1, Diego Ivan Caviedes2, Daniel Ricardo Delgado3
Los parámetros de solvatación preferencial por etanol (EtOH) de 4-hidroxi-2,5-dimetil-3 (2H) -furanona (HDMF) fueron derivados de sus propiedades termodinámicas de solución por medio de los métodos de las integrales inversas de Kirkwood-Buff y cuasi-reticular -cuasi-químico (QLQC). Según el método IKBI, el parámetro de solvatación preferencial δx1,3 del EtOH es negativo en mezclas ricas en agua pero positivo en mezclas de ricas en etanol. Posiblemente la hidratación hidrofóbica alrededor de los grupos metilo de la HDMF juega un papel relevante en la solvatación en mezclas ricas en agua. La mayor solvatación por parte del EtOH en mezclas ricas en etanol pude ser debido principalmente a los efectos de polaridad y comportamiento ácido de los grupos hidroxilo del compuesto frente a los disolventes más básicos presentes en las mezclas. De otro lado, según el método QLQC, este compuesto es solvatado preferentemente por el cosolvente en la mayoría de las mezclas de sistema de agua etanol.
Palabras clave: HDMF; etanol; solvatación preferencial; integrales inversas de Kirkwood-Buff; cuasi-reticular-cuasi-químico.
The parameters of preferential solvation of 4-hydroxy-2.5-dimetyl-3 (2H)-furanone (HDMF) in ethanol (EtOH) were derived from its thermodynamic properties of solution by means of inverse Kirkwood-Buff integrals and quasi-lattice-quasi-chemical (QLQC) methods. According to the IKBI method, the preferential solvation parameter δx1,3 of EtOH is negative in water-rich mixtures but positive in ethanol-rich mixtures. It is possible that the hydrophobic hydration around the methyl groups of the HDMF plays a role in the solvation in water-rich mixtures. The greatest EtOH solvation in ethanol-rich mixtures may have been due, mainly, to the effects of polarity and acid-base behavior of the hydroxyl groups in the compound against the most basic solvents in the solution. On the other hand, using the QLQC method, this compound is preferably solvated by the cosolvent in most of the water-ethanol system mixtures.
Key words: HDMF; ethanol; preferential solvation; inverse Kirkwood-Buff integrals; quasi-lattice-quasi-chemical
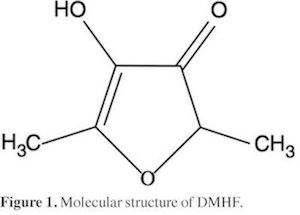
DMHF, is an important aroma compound which has been identified in many fruits, e.g. pineapples (Rodin et al., 1965), strawberries (Ohloff, 1969), arctic bramble (in trace) (Kallio, 1976), raspberries (Honkanen et al., 1980), as an off-flavour in aged orange juice (Tatum et al.,1975), and in experimental hybrids of German wine (Rapp et al., 1980). It occurs in cooked, roasted and fermented food materials, e.g. beef broth (Tonsbeek et al, 1968), roasted filberts ,d roasted almond (Sheldon et 1972), coffee and soya sauce (Tressl etal., 1978). DMHF ( Fig. 1, IUPAC 4-hydroxy-2,5-dimethyl-3(2H)-furanone, DMHF, C6H803, CAS Registry No. 3658-77-3).
Solubility determination of drugs and similar compounds in all possible co-solvent mixtures is very important for pharmaceutical and chemical scientists involved in several development stages such as substances purification and design of liquid medicines (Jouyban, 2010). Co-solvency has been employed in pharmacy to increase solubility of drugs to develop homogeneous pharmaceutical liquid dosage forms long time ago (Rubino, 1988; Yalkowsky, 1999; Jouyban, 2010). In this way, an investigation have been carried out to evaluate the effect of composition and temperature on the solubility of DMHF in ethanol + water (Wang et al., 2015). Nevertheless, the drug preferential solvation by the solvents, i.e. the cosolvent specific composition around the DMHF molecules has not been studied. It is important to note that ethanol is the most widely used co-solvents to develop liquid medicines (Aulton, 2002); moreover, they are also used as additives in several kinds of industrial foods (Smolinske, 1992).
The inverse Kirkwood-Buff integrals (IKBI) are a powerful tool for evaluating the preferential solvation of non-electrolyte compounds in co-solvent mixtures, describing the local compositions around the solute with respect to the different components present in the solvent mixture (Marcus, 2002, 2009, 2013). Applied to the present research, this treatment depends on the values of the standard molar Gibbs energies of transfer of DMHF from neat water to the co-solvent + water mixtures and the excess molar Gibbs energy of mixing for the binary mixtures free of drug. In similar way, quasi-lattice quasi-chemical (QLQC) approach is also useful to do evaluate preferential solvation although is not too much exact as IKBI approach is. This method supposes that the number of nearest neighbors a molecule has (the lattice parameter Z) is the weighted mean of the lattice parameter of the pure components. It also presumes that the interaction energy of a molecule of any component with others is independent of the nature of the neighbors. The model also assumes that ideal volumes and entropies of mixing take place. The main advantage of this method is that non-derivative functions are required as they are in the case of the IKBI method (Marcus, 2002, 2009, 2013).
In this paper the IKBI and QLQC approaches are applied to evaluate the preferential solvation of DMHF in the binary mixtures conformed by ethanol (EtOH) and water (W). QLQC is applicable in both systems because the maximum solubility is obtained in the neat co-solvent (Wang et al., 2015). The results are expressed in terms of the preferential solvation parameter (δx1,3) of the solute by the co-solvent, ethanol according to the mixtures composition. Thus, this study is similar to that developed by analyzing the behavior of the different drugs in some co-solvent + water mixtures (Jiménes et al., 2015; Muños et al., 2015; Cárdenas et al., 2014; Delgado and Martinez, 2015,2014a; Peña et al., 2014a; Delgado et al;2014a,2014b, 2013a, 2013b, 2011; Cristancho et al., 2013; Ruidiaz et al., 2010).
According to IKBI method, the solvation results are expressed in terms of the preferential solvation parameter δx1,3 of the solute by the co-solvent (compound 1) according to the following expression:
Where x1 is the mole fraction of co-solvent in the bulk solvent mixture and xL1,3 is the local mole fraction of co-solvent in the environment near to the drug. If δx1,3 > 0 then DMHF is preferentially solvated by cosolvent; on the contrary, if it is < 0 the drug is preferentially solvated by water. Values of δx1,3 are obtainable from of the Kirkwood-Buff integrals, G13, and these, in turn, from thermodynamic data of the co-solvent mixtures with the solute dissolved on it, as shown in equations 2 and 3 (Marcus, 2008,2009):
Where kT is the isothermal compressibility of the cosolvent + water solvent mixtures (in GPa-1), V̄ , and V̄2 are the partial molar volumes of the solvents in the mixtures (in cm3 mol-1), similarly, V̄3 is the partial molar volume of solute in these mixtures (in cm3 mol-1). The function D is the derivative of the standard molar Gibbs energies of transfer of the drug (from neat water to co-solvent + water mixtures) with respect to the solvent composition (in kJ mol-1, as also is RT) and the function Q involves the second derivative of the excess molar Gibbs energy of mixing of the two solvents ( ) with respect to the water proportion in the mixtures (also in kJ mol-1) (Marcus, 1998,2008,2009). These quantities are calculated according to equations (4) and (5).
) with respect to the water proportion in the mixtures (also in kJ mol-1) (Marcus, 1998,2008,2009). These quantities are calculated according to equations (4) and (5).
The preferential solvation parameter of DMHF by the co-solvent can be calculated from the Kirkwood-Buff integrals as follows (Marcus, 2008, 2009):
Here Vcor is the correlation volume which is obtained by using (Marcus, 2008,2009):
where r3 is the radius of DMHF (expressed in nm). However, the definitive correlation volume requires iteration, because it depends on the local mole fractions around the solute. This iteration is done by replacing δx1,3 in the equation (1) to calculate em>xL1,3 until a nonvariant value of Vcor is obtained.
For the QLQC method, the local mole fraction of cosolvent around the DMHF molecules is defined as (Marcus, 2008):

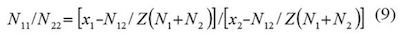
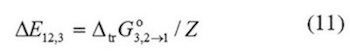
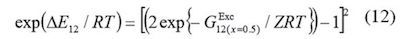
In these equations, the lattice parameter Z is usually assumed as 10. N1 and N2 are the number of molecules of both components in the bulk, whereas, N11,N22, and N12 are the number of neighboring pairs of these molecules in the quasi lattice. Equation (10) expresses the difference in the molar neighbor interaction energies of DMHF with the co-solvent and water, ΔE123, by the molar Gibbs energy of transfer from water to co-solvent per neighboring lattice. DE12 denotes the molar energy of interaction of solvent on neighboring quasi-lattice sites. It is important to keep in mind that just the Gibbs energy of the solute transfer between the neat solvents and the excess Gibbs energy of mixing at equimolar composition of both solvents are required for this method.
Standard molar Gibbs energy of transfer of DMHF from neat water to co-solvent + water mixtures (Table 1) was calculated and correlated to non-regular polynomials by using the equation 13 from the drug solubility data reported previously in the literature (Wang etal., 2015). Thus, the coefficients of the polynomials are shown in Table 2.
D values are calculated from the first derivative of the polynomial models solved according to the co-solvent mixtures composition. This procedure was done vaiying by 0.05 in mole fraction of co-solvent.
Q and RT kT values, as well as the partial molar volumes of co-solvents and water in these binary mixtures were taken from the literature (Delgado and Martínez, 2014b; Jiménez, 2014). Otherwise, as it is well known, the partial molar volumes of nonelectrolyte drugs are not frequently reported in the literature. For this reason, as was made previously with other drugs in similar studies (Delgado 2014c), the molar volume of DMHF is considered here as independent of co-solvent composition and temperature. Thus, the V3 value presented in Table 3 was calculated according to the groups contribution method proposed by Fedors (1974). Furthermore, the molecular radius was also calculated from the respective molar volume by using the equation (14), where NAv is the Avogadro number, as 0.3483 nm.
On the other hand, Table 4 shows that the G13 and G23 are negative in all compositions at all temperatures considered indicating that this compound has affinity for both organic and aqueous media.
In order to use the IKBI method, the correlation volume was iterated by using the equations 1, 6 and 7 to obtain the values. Vcor is almost independent on temperature in water-rich mixtures but it increases in some extent in co-solvent-rich mixtures. This behavior is proportional to the increase in molarvolume of the respective co-solvent mixtures with the temperature (Jiménez et al., 2004; Jiménez and Martínez, 2005).
DMHF could act in solution as a Lewis acid due to the hydrogen atoms in its -OH groups (Fig. 1) in order to establish hydrogen bonds with proton-acceptor functional groups in the solvents (oxygen atoms in -OH groups). In addition, this compound could act as a Lewis base due to free electron pairs in oxygen atoms of —O—, >C=0, or -OH groups (Fig. 1), to interact with acidic hydrogen atoms in water and EtOH.
According to IKBI method the values of δx1,3 vary non-linearly with the co-solvent proportion in the aqueous mixtures ( Table 5, Figs. 2). Addition of cosolvent to water tends to make negative the δx1,3 values of DMHF from the pure water up to the mixture X1 = 0.25 in both systems reaching minimum values near to x1 = 0.05. Possibly the structuring of water molecules around the non-polar groups of this dmg (Fig. 1) contributes to lowering of the net δx1,3 to negative values in these water-rich mixtures. IKBI δx1,3 values are higher again in EtOH + water.
In the mixtures with composition 0.25 < x1 < 1.00, the local mole fractions of co-solvent are greater than those for water. In this way, the co-solvent action may be related to the breaking of the ordered structure of water (aggregates stabilized by hydrogen bonding) around the non-polar moieties of DMHF, which could increase the solvation, exhibiting maximum values near to x1 = 0.70.
According to the preferential solvation results, it is conjecturable that in intermediate composition and in co-solvent-rich mixtures, DMHF is acting as Lewis acid, because these co-solvents are more basic than water, i.e. the Kamlet-Taft hydrogen bond acceptor parameters are Β = 0.75 for EtOH, and 0.47 for water (Kamlet and Taft, 1976; Marcus, 1998).
On the other hand, in order to use the QLQC method, the excess Gibbs energy of mixing values of the equimolar mixture of EtOH + water mixtures was calculated according to some equations reported in the literature (Marcus, 1998; Delgado and Martinez, 2014a. According to the QLQC method (Table 5 and Fig. 2), DMHF is preferentially solvated by the co-solvent in all the mixtures between 293.95 and 308,15 K, the other hand at 313,15 K DMHF is preferentially solvated by the EtOH from near water at 0.85 in molar fraction of EtOH and solvated by the water from 0.85 in molar fraction of EtOH at near EtOH.
The QLQC method show higher δx1,3 values in water-rich mixtures unlike the method the IKBI method which reports greater values in ethanol rich mixtures. Maximum values are found in the mixture with x1 = 0.70 EtOH + water mixtures. It is important to note that, as has been indicated in the literature, the IKBI method is more adequate than QLQC method in order to discriminate the effect of the co-solvent composition on the local mole fraction of the solvents around the drug molecules, in particular in the water-rich mixtures (Delgado et al., 2014a; Peña al., 2014). Nevertheless , it is important to keep in mind that QLQC only requires the Gibbs energy of transfer of DMHF from water to co-solvent and the excess Gibbs energy of mixing in the co-solvent mixture with composition X1 = 0.50, and therefore, it is more easy to use.
According to IKBI method DMHF is preferentially solvated by water in water-rich mixtures but preferentially solvated by co-solvent in mixtures with compositions from x1 = 0.25 to neat co-solvent in EtOH + water at all temperatures considered. It is possible that the hydrophobic hydration around methyl groups of DMHF plays a relevant role in the solvation in water-rich mixtures. The more solvation by co-solvents in mixtures of similar composition and co-solvent-rich mixtures could be due to basic behavior of DMHF in front to water, which is the more acidic solvent. On the other hand, according to the QLQC method, this compound would be preferentially solvated by the cosolvents in all the possible mixtures. Nevertheless, it is important to consider that the IKBI method is more rigorous than QLQC and more reliable results are thus obtained with the former method. Finally, it is noteworthy that these treatments contribute to the understanding of the chemical behavior of pharmaceutical and food components in complex solutions.
1. Aulton, M.E., 2002. Pharmaceutics, The Science of Dosage Forms Design, 2nd edn. London: Churchill Livingstone.
2. Cárdenas, ZJ., Jiménez, D.M., Martínez, L, 2014. Preferential solvation of ketoprofen in some co-solvent binary mixtures. J. Solut. Chem. 43, 1904-1915.
3. Cristancho, D.M., Delgado, D.R., Martínez, F., 2013. Preferential Solvation of Ethylhexyl Triazone in Ethyl Acetate + EtOH Mixtures According to the Inverse Kirkwood-Buff Integrals Method. Lat. Am. J. Pharm. 32 (10) 1538-1545.
4. D.R., Holguin, A.R., Almanza, O.A., Martínez, F., Marcus, Y., 2011. Solubility and preferential solvation of meloxicam in ethanol + water mixtures. Fluid Phase Equilib. 305, 88-95
5. Delgado, D.R., Martínez, F., 2015. Preferential solvation of some structurally related sulfonamides in 1-propanol + water co-solvent mixtures. Phys. Chem. Liq. 53(3), 293-306.
6. Delgado, D.R., Martínez F., 2014. Preferential solvation of sulfadiazine, sulfamerazine and sulfamethazine in ethanol + water solvent mixtures according to the IKBI method. J. Mol. Liq. 193, 152-159.
7. Delgado, D.R., Martínez, F., 2014. Solubility and preferential solvation of sulfadiazine in methanol + water mixtures at several temperatures. Fluid Phase Equilib. 379,128-138.
8. Delgado, D.R., Peña, M.A., Martínez, F., 2014. Preferential solvation of some sulfonamides in 1,4-dioxane + water co-solvent mixtures at 298.15 K according to the inverse Kirkwood-Buff integrals method. Rev. Acad. Colomb. Cienc. 38(146), 104-114.
9. Delgado, D.R., Peña, M.A., Martínez, F., 2014. Preferential Solvation of Some Sulfonamides in Propylene Glycol + Water Solvent Mixtures According to the IKBI and QLQC Methods. J. Solution Chem. 43, 360-374.
10. Delgado, D.R., Peña, M.A., Martínez, F., 2013. Preferential solvation of acetaminophen in ethanol + water solvent mixtures according to the inverse Kirkwood-Buff integrals method. Rev. Colomb. Cienc. Quim. Farm. 42(2), 298-314.
11. Delgado, D.R., Vargas, E., Martínez, F., 2013. Preferential solvation of xylitol in ethanol + water co-solvent mixtures according to the IKBI and QLQC methods. Rev. Colomb. Quim. 42(1), 59-66.
12. Fedors, R. F., 1974. A method for estimating both the solubility parameters and molar volumes of liquids. Polym. Eng. Sci. 14,147-154.
13. Honkanen,E.,Pyysalo,T., Hirvi,T., 1980. The aroma of Finnish wild raspberries, Rirbus idaeirs L. Z. Lebensm. Unters. Forsch. 171, 180-182.
14. Jiménez, J., Manrique, J., Martínez, F., 2004. Effect of temperature on some volumetric properties for ethanol + water mixtures. Rev. Colomb. Cienc. Quim. Farm. 33, 145-155.
15. Jiménez, J., Martínez, F., 2005. Study of some volumetric properties of 1,2-propanediol + water mixtures at several temperatures. Rev. Colomb. Cienc. Quim. Farm. 34, 46-57.
16. Jiménez, D.M., Cárdenas, Z.J., Delgado, D.R., Martínez, F., Jouyban, A., 2014. Preferential solvation of methocarbamol in aqueous binary cosolvent mixtures at 298.15 K. Phys. Chem. Liq. 52, 726-737.
17. Jiménez, D.M., Cárdenas, Z.J., Delgado, D.R., Peña, M.A., Martínez, F., 2015. Solubility temperature dependence and preferential solvation of sulfadiazine in 1,4-dioxane + water co-solvent mixtures. Fluid Phase Equilib. 397, 26-36.
18. Jouyban, A., 2010. Handbook of Solubility Data for Pharmaceuticals. CRC Press, Boca Raton (FL).
19. Kallio, H., 1976. Identification of vacuum steam distilled aroma components in the press juice of arctic bramble, Rubus arcticus, L. J. Food. Sci. 41, 555-562.
20. Kamlet, M.J.,Taft, R. W., 1976. The solvatochromic comparison method. I. The beta-scale of solvent hydrogen-bond acceptor (HBA) basicities. J. Am. Chem. Soc. 98, 377-383.
21. Marcus, Y., 1998. The Properties of Solvents. John Wiley & Sons, Chichester.
22. Marcus, Y., 2002. Solvent Mixtures: Properties and Selective Solvation. Marcel Dekker, Inc., New York.
23. Marcus, Y., 2008. On the preferential solvation of drugs and PAHs in binary solvent mixtures. J. Mol. Liq. 140,61-67.
24. Marcus, Y., 2009. Preferential solvation of ibuprofen and naproxen in aqueous 1,2-propanediol. Acta Chimica Slovenica, 56, 40-44.
25. Marcus, Y., 2013. Preferential solvation in mixed solvents. In: Smith, P. E., Matteoli, E., O’Connell, J. P. (Eds.). Fluctuation Theory of Solutions: Applications in Chemistry, Chemical Engineering, and Biophysics. CRC Press, Taylor & Francis Group, Boca Raton.
26. Muñoz, M., Delgado, D.R., Peña, M.A., Jouyban, A., Martinez, F., 2015. Solubility and preferential solvation of sulfadiazine, sulfamerazine and sulfamethazine in propylene glycol + water mixtures at 298.15 K. J. Mol. Liq. 204, 132-136.
27. Ohloff, G, 1969. Chemie der Geruchs-und Gesch-niacksstoffe. Fortschr. Chem. Forsch. 12, 185-259.
28. Peña, M.A., Delgado, D.R., Martinez, F., 2014. Preferential Solvation of Acetaminophen in Propylene Glycol + Water Co-Solvent Mixtures. J Appl Sol Chem Model. 3, 65-73.
29. Rapp. A., Knipser, W., Engel, F., Ullemeyer, H., Heimann, W., 1980. Fremdkomponenten im Aroma von Trauben und Weinen interspezifischer Rebsorten: I. Die Erd be ernote. Vitis. 19, 13-23.
30. Rodin, J.O., Himel, C.M., Silverstein, R.M., Deeper, R.W., Gortner, W.A., 1965. Volatile flavor and aroma components of pineapple. I. Isolation and tentative identification of 2,5-dimethyl-4-hydroxy-3(2H)-furanone. J. Food. Sci. 30, 280-285.
31. Rubino, J.T., 1988. Cosolvents and cosolvency. In: Swarbrick, J., Boylan, J.C. (Eds.). Encyclopedia of Pharmaceutical Technology. Marcel Dekker, Inc.,Vol 3, New York.
32. Ruidiaz, M.A., Delgado, D.R., Martinez, F., Marcus, Y., 2010. Solubility and preferential solvation of indomethacin in 1,4-dioxane + water solvent mixtures. Fluid Phase Equilib. 299, 259-265.
33. Sheldon, R.M., Findsay, R.C., Fibbey, F.M., 1972. Identification of volatile flavor compounds from roasted filberts. J. Food. Sci. 3, 13-316.
34. Smolinske, S.C., 1992. Handbook of Drug, Food and Cosmetic Excipients. CRC Press EEC, Boca Raton.
35. Tatum, J.H., Nagy, S., Berry, R.E., 1975. Degradation products formed in canned single-strength orange juice during storage. J. Food. Sci. 40, 707-709.
36. Tonsbeek, C.H.T., Plancken, A.J., Weerdhof, T.v.d., 1978. Components contributing to beef flavor. Isolation of 4-hydroxy-5-methyl-3(2H) furanone and its 2,5-dimethyl homolog from beef broth. J. Agrie. Food Chem. 16, 101-1021.
37. Tressl, R., Bahri, D., Koppler, H., Jensen,A., 1978. Diphenole und Caramelkomponenten in Rostkaffees verschiedener Sorten. Z. Febensm. Unters. Forsch. 16, 111-114.
38. Wang, F-Y., Fia, X-C., Zhua, F., Shaa, Z-F., Wang, Y-F., Yang, F-B., 2015. Experimental determination and correlation of the solubility of 4-hydroxy-2,5-dimethyl-3(2H)-furanone (DMHF) in binary (ethanol + water) solvent mixtures. J. Mol. Fiq. 208,211-218.
39. Yalkowsky, S.H., 1999. Solubility and Solubilization in Aqueous Media. American Chemical Society and Oxford University Press, New York.