Artículo de Investigación
R.F.S Revista Facultad de Salud Enero-Junio de 2016;8(1): 9-15
Federico Perdomo-Celis1 2, Doris M. Salgado1,2, Carlos F. Narváez1*
1 Semillero de formación SINEDIR, Grupo de Parasitología y Medicina Tropical, Programa de Medicina, Universidad Surcolombiana, Neiva, Colombia.
2 Departamento de Pediatría, Hospital Universitario de Neiva, Colombia
* Correspondencia: Dr. Carlos Femando Narváez. Correo electrónico: cfnarvaez@usco.edu.co
Recibido: 27/11/2015
Revisado: 22/02/2016
Aceptado: 17/05/2016
Dengue is an important viral vector-borne disease worldwide. Its diagnosis requires laboratory confirmation and the detection of dengue virus (DENV)-specific immunoglobulin (lg) M by capture enzyme-linked immunosorbent assay (ELISA) is a method frequently used. However, commercial kits of IgM-capture ELISA for dengue diagnosis are expensive not easily available and obtain the results take long time in endemic areas. Here, we aimed to standardize an in-house capture ELISA for the detection of DENV-specific IgM in plasma for diagnosis of DENV infection. Plasma from two children with confirmed acute DENV-2 infection was evaluated and two mouse monoclonal anti-DENV antibodies (one type-specific and the other one serotype-cross-reactive) were tested. The assay was effective in the detection of DENV-specific IgM in plasma and was comparable with a commercially available kit, even at the highest dilutions of the sample and the mouse anti-DENV antibodies. Furthermore, in a child with primary DENV-2 infection, plasma anti-DENV IgM was found to be serotype-specific. This work strengthens the technical capacity for the study and diagnosis of DENV infection in an endemic area.
Keywords: Dengue, ELISA, plasma, immunoglobulin M, cross-reactive.
El dengue es una importante enfermedad transmitida por vectores a nivel mundial. Su diagnóstico requiere confirmación por laboratorio. La detección de inmunoglobulina (lg) M específica de virus dengue (DENV) por ensayo de inmunoadsorción ligado a enzima (ELISA) es un método frecuentemente usado. Sin embargo, las pruebas comerciales de ELISA de captura IgM para el diagnóstico de dengue son costosos, no están fácilmente disponibles y la entrega de resultados toma más del tiempo que el deseado en áreas endémicas. Aquí se propuso desarrollar una captura de ELISA para detección de IgM específica de DENV en plasma y ayudar al diagnóstico de dengue. El plasma de dos niños con infección aguda por DENV-2 confirmada y dos anticuerpos monoclonales anti-DENV hechos en ratón (uno serotipo-específico y el otro de reacción cruzada para los 4 serotipos) fueron evaluados. El ensayo fue efectivo en la detección de IgM específica de DENV y es comparable con un estuche comercialmente disponible, incluso a las mayores diluciones de la muestra y de los anticuerpos anti-DENV. Adicionalmente, en un niño con infección primaria por DENV-2, la IgM específica de DENV en plasma fue serotipo-específica. Este trabajo fortalece la capacidad tecnológica para el estudio y diagnóstico de la infección por DENV en un área endémica.
Palabras clave: Dengue, ELISA, plasma, inmunoglobulina M, cross-reactivo.
Dengue is an important viral vector-borne disease, with an annual prevalence of 390 million of infections, 96 million of these symptomatic and 500,000 cases of clinically severe forms(1\ This disease is caused by any of four dengue virus (DENV) serotypes (DENV-1,2,3 and 4), and it can clinically range from asymptomatic to potentially life-threatening disease, with shock, bleeding and organ dysfunctional The diagnosis is based in clinical and laboratory criteria, the latter including direct methods (detection of viral components such as proteins and nucleic acids) and indirect methods (detection of virus-specific immunoglobulins).
These assays are positive during different phases of the disease, correlating with the early presence of the virus in blood and the later induction of humoral immune response (before and after the fourth day of beginning of the symptoms, respectively(3,4). After the initial increase of DENV-specific IgM during a primary infection, it remains detectable up to three months(5)
The capture enzyme-linked immunosorbent assay (ELISA) for the detection of DENV-specific IgM is frequently used for dengue diagnosis, however commercial kits that use this ELISA format are expensive and inaccessible in some endemic areas, limiting the application of the assay for rapid diagnosis of infection and for research and medical purposes. To overcome this problem, some laboratories have designed capture ELISAs for the detection of DENV-specific IgM, with acceptable sensitivity and specificity(6,7). Therefore, the development and implementation of these assays in highly endemic areas would be essential for the diagnosis, in addition to support local epidemiological and immunological studies. Here, we standardized a semiquantitative capture ELISA for the detection of DENV-specific IgM in plasma, which was effective and could be a helpful tool to dengue studies when combined with other direct and indirect methods.
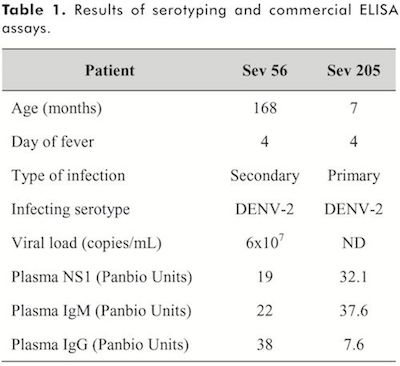
Patients and samples. This study was approved by the Ethics Committee of Universidad Surcolombiana and Hospital Universitario of Neiva. Two children with acute DENV infection were included according to WHO 2009 revised guidelines(2). Infection was confirmed by detection of non-structural protein (NS)-l and/or DENV-specific IgM by ELISA, and the infecting serotype was determined by conventional reverse transcription polymerase chain reaction (RT-PCR), as described below. Written parents informed consent was obtained from each of the children included. All experiments followed the principles expressed in the Declaration of Helsinki. Socio-epidemiological characteristics of the included children are shown in Table 1. Two to four milliliters of venous blood was collected in tubes containing ethylenediaminetetraacetic acid (EDTA, BD Vacutainer®; Ref: 367861). The tubes were centrifuged at 300 X g, and the plasma was collected and stored at ?70°C until the time of analysis.
Viruses. DENV-1,2,3 and 4 were propagated in Vero-76 cells (acquired from ATCC, number CRL-1587D). Five hundred juL of each serotype (kindly provided by Dr. Ivan Dario Velez, Universidad de Antioquia, Colombia) were added to a Vero-76 cells monolayer with a 70% confluence and they were incubated in IX DMEM medium with 2% fetal bovine serum (both from GIBCO®, Carlsbad, CA; Cats: 11965-126 and 16000-044, respectively) at 37°C, 5% C02. After 5 days culture, the appearance of cytopathic effect on the cell monolayer was checked by inverted light microscopy and the culture supernatant (cell lysate) was collected, centrifuged and frozen at -70°C until use. As control, cell lysate from uninfected cells (mock) treated identically as described above was also collected. The purity of each serotype was checked by RT-PCR, as described below.
Mouse anti-DENV antibodies for capture ELISA. Two anti-DENV antibodies were tested: mouse anti-E protein antibody (clone 3H5.1, IgGp immunogen: DENV-2; stock concentration lmg/mL; Millipore, Billerica, MA, Cat: MAB8702) with reported specificity for DENV-2(8), and mouse anti-DENV complex antibody (clone M8051125, IgG2a, immunogen: DENV-4; stock concentration lmg/mL; Fitzgerald, Acton, MA, Cat: 10-D35), reported to be serotype-cross reactive(9).
Detection and quantitation of DENV in cell lysate and plasma by conventional RT-PCR and real time PCR. Infecting serotypes in the analyzed plasma samples and cell lysate were determined by RT-PCR and real time PCR, respectively, as previously described(10,11) The mean of the number of copies/mL of DENV-2 ribonucleic acid (RNA) in cell lysate was 4.9 x 10 (11) as we previously reported (10)
Commercial ELISA kits for diagnosis of DENV infection. For the detection ofDENV-specific IgM and IgG and viral NS1 protein, Dengue IgM and IgG capture ELISA (Refs: E-DEN01M and E-DEN02G, respectively) and Dengue Early ELISA (Ref: E-DEN02P) were used (all from Panbio®, Alere, Australia), following the manufacturer’s instructions and as previously described(10). The type of infection (primary or secondary) was determined by the DENV-specific IgM/IgG ratio in plasma, taking a ratio ? 2 as a secondary infection, as previously reported (12)
Capture ELISA for the detection of DENV-specific IgM. Nunc MaxiSorp® flat-bottom 96 well plates (NUNC, Cat: 44-2404-21) were coated with 70?L per well containing goat affinity purified polyclonal F(ab’)2 fragment to Human IgM heavy chains (KPL, Gaithersburg, MD, Cat:201-1003) at a concentration of 2?g/mL in IX Dulbecco’s phosphate-buffered saline (DPBS, Gibco®, Carlsbad, CA; Ref: 14190-144), pH 7.3, and incubated overnight at 4°C. Then, the plates were blocked for lh at 37°C with 1507L per well containing 5% nonfat milk in IX DPBS (Gibco®, Carlsbad, CA; Cat: 21600-069) with 0.05% Tween 20 (ACROS, Geel, Belgium, Cat. 23336-2500) (5% blocking solution). Seventy microliters per well of plasma samples at specified dilutions in 2.5% blocking solution and negative controls (2.5% blocking solution alone) were added and incubated for 2h at 37°C. Afterwards, plates were washed four times with 0.1% Tween 20 in IX PBS (wash buffer) and 70?L per well containing the indicated DENV serotype or mock cell lysate at a final dilution of 1/10 in 2.5% blocking solution were added and the plate incubated for lh at 37°C. Subsequently, plates were washed four times and 70?L per well containing mouse anti-DENV-2 antibody or mouse anti-DENV complex antibody at concentrations of 10, 2 and l?g/mL in 2.5% blocking solution were added and the plates were incubated for lh at 37°C. Next, 70?L per well containing biotin-labeled affinity purified goat anti-mouse IgG antibody (KPL, Gaithersburg, MD, Cat: 16-18-02) and horseradish peroxidase labeled streptavidin (KPL, Gaithersburg, MD, Cat: 14-30-00), both at a final concentration of 0.5?g/mL in 2.5% blocking solution, were added respectively, each one being incubated for lh at 37°C. Finally, plates were revealed with 70?L per well containing tetramethylbenzidine 0.4g/L and 0.2% H202 (TMB, KPL, Gaithersburg, MD, Cat: 50-76-00) and reaction was stopped with 17.57L per well containing 2M H2S04 (MERCK, Darmstadt, Germany, Cat: K38346532). Plates were read at 450nm in a Multiskan FC photometer (Thermo, Waltham, MA). Dilutions with optical density double or higher than the negative control and mock-treated wells were considered positive.
Statistical analysis. GraphPad Prism® 6.0 (GraphPad Software, La Jolla, CA) software was used for the statistical analyses. Data are presented as mean and the respective standard deviations. Associations between variables were determined with the Pearson test. A P value <0.05 was taken as significant.
Patients included. Two children of 7 and 168 months old hospitalized at Departamento de Pediatría Hospital Universitario of Neiva, Colombia, were included. Both cases were clinically classified as severe dengue according to the revised WHO guidelines, 2009(2). Venous blood samples were taken on fourth day of fever. One patient presented primary infection and the other one had secondary infection, determined by DENV-specific IgM/IgG ratio as described in methods section. Both patients were infected by DENV-2, as determined by RT-PCR. The results of laboratory assays are described in Table 1.
Determination of working concentration of mouse anti-DENV antibodies. For the standardization of capture ELISA, plasma from a child with secondary infection by DENV-2 was used (as shown in Table 1). Previously optimized working concentrations were used for F(ab’)2 fragment to Human IgM heavy chains, biotin-labeled affinity purified goat anti-mouse IgG antibody and horseradish peroxidase labeled streptavidin(13), therefore only several concentrations of mouse anti-DENV antibodies were evaluated. As shown in the Figure 1, we assessed 10, 2 and ljag/mL of mouse anti-DENV-2 antibody and mouse anti-DENV complex antibody in 2.5% blocking solution, with previous addition of DENV-2 or mock at a final dilution of 1/10 in 2.5% blocking solution. As additional negative control, 2.5% blocking solution without sample was added. As shown in the Figure 1, in comparison with the mock and the negative control, at least the double of optical densities (OD 450nm) were obtained when DENV-2 was added, and they were dose-dependent, with a strong negative correlation between OD450nm and plasma dilutions for all the concentrations of mouse monoclonal antibodies tested (Pearson r=-l, P=0.01). The concentrations of anti-DENV-2 antibody evaluated (clone 3H5.1) and anti-DENV complex antibody (clone M8051125) showed similar OD450nm dynamic (Figure 1A and 1B, respectively), therefore working concentration of 1 μg/mL was selected for further experiments. In the Table 2 are depicted the OD450nm for each dilution and the signal / background ratio. As expected, we confirmed that anti-DENV complex antibody is cross-reactive (Figure 1B), as its original immunogen was DENV-4 and here this monoclonal antibody also was reactive against DENV-2. In summary, efficient detection of DENV-specific IgM by capture ELISA was obtained at high dilutions of anti-DENV antibodies, and serotype-cross-reactivity of anti-DENV complex antibody was confirmed.
Effectiveness of in-house DENV-specific IgM capture ELISA. To test the efficiency in the detection of DENV-specific IgM in plasma, the performance of our in-house assay and a commercially available ELISA kit was compared on a plasma dilution of 1/100. Consistent with commercial kit results, the patients were also identified as DENV-specific IgM positive when the in-house assay was used, both with mouse anti-DENV2 antibody (clone 3H5.1) and mouse anti-DENV complex monoclonal antibody (clone M8051125) (Figure 2). Furthermore, comparable OD450nm signal / OD450nm background ratio was found between the assays (Figure 2). Together, these results support an adequate performance of the locally standardized assay.
DENV serotype-specific IgM was detected in primary infection. The capture ELISA was effective in the detection of DENV-specific IgM in secondary infection, and then we evaluated its performance in the detection of DENV-specific IgM in plasma from a child with primary DENV-2 infection. In this case, to test if IgM generated against DENV is serotype-specific or cross-reactive, both DENV-2 and a DENV-1/4 mix were used in the assay before the addition of mouse anti-DENV antibodies at the optimized working concentration. As shown in Figure 3, compared to the mock and the negative control, at least the double of OD450nm were obtained when plates were treated with DENV-2 but no with DENV-1/4 mix, both in the presence of mouse anti-DENV2 antibody (clone 3H5.1) or mouse anti-DENV complex monoclonal antibody (clone M8051125) (Figure 3A and B, respectively), with high signal / noise ratios only when DENV-2 was the virus added (Table 2), and there was a strong negative correlation between OD450nm and plasma dilutions (Pearson r=-1, P=0.01). Thus, plasma DENV-specific IgM from a child with primary DENV-2 infection bound preferentially to its currently infecting serotype, and had low or no cross-reactivity to DENV-1 or 4.
Here we evaluated the performance of a capture ELISA for the detection of DENV-specific IgM in plasma, both in primary and secondary infections, showing to be effective. Moreover, IgM in plasma from a child with primary infection was serotype-specific.
For the standardization of the capture ELISA, a plasma sample from a child with secondary infection by DENV-2 in his fourth day of fever was used (Table 1), according to previous reports which showed that DENV-specific IgM is generated early in secondary infections(14). Although serum is frequently used for serological assays, its use does not allow cellular analyses for which anticoagulated blood is required. Therefore, plasma was preferred over serum for our standardization. Secondary mouse anti-DENV antibodies were evaluated, as in this format of ELISA the detection of plasma DENV-specific IgM depends on them. Indeed, the mouse anti-DENV complex antibody (clone M8051125), with reported and confirmed serotype-cross reactivity (Figure 1B), was included to assess if IgM generated against DENV infection is type-specific or serotype-cross-reactive.
Both mouse anti-DENV2 (type-specific) and mouse anti-DENV complex antibody were evaluated at three concentrations (10,2 and 1&mu/mL, Figure 1). One advantage of this capture ELISA is that it is a semiquantitative assay. Here, we used two-fold serial dilutions of plasma samples and, in comparison with mock-treated wells and the negative control, DENV-2-treated wells had higher OD450nm (at least the double of the mock-treated wells and negative control) even at the higher plasma dilution (1/800) (Figure 1), indicating that in this assay probably the limiting titer of DENV-specific IgM was not obtained, but suggests a good sensitivity thereof. Moreover, at the lowest concentrations of monoclonal antibodies (1μg/mL), both mouse anti-DENV antibodies showed similar OD450nm than those of the highest concentration (Figure 1), reason why this was selected for this capture ELISA. Although we only used one plasma sample to standardize the assay, the dose-effect response observed after the serial dilutions supports our observations. Another advantage of our indirect format is the flexibility in the use of different detecting antibodies (in this case a biotin-labeled goat anti-mouse IgG) and to avoid the decreased affinity of secondary antibodies that can be induced after enzyme conjugation (15).
The capability of the in-house capture ELISA for the detection of DENV-specific IgM in plasma was compared with a commercially available ELISA on a plasma dilution of 1/100, which is recommended by this kit’s manufacturer. When the performance of the in-house assay was evaluated qualitatively (number of DENV-specific IgM positive or negative plasma samples) and quantitatively (OD450nm signal / OD450nm background ratio), it showed to be comparable to the commercial kit (Figure 2), supporting its efficiency and usefulness in dengue diagnosis. Of note, the anti-DENV antibody in the Panbio® ELISA kit is a horseradish peroxidase conjugated monoclonal antibody that is pre-mixed with DENV antigens before the addition to the plate, but its reactivity is not described by the manufacturer. However, as in our capture ELISA a biotin-streptavidin detection system was used, we expected to obtain higher OD450nm than the commercial kit, which was not observed. The distinct reactivity and affinity of the monoclonal antibodies here used could explain this finding.
Based on the effectiveness obtained with this in-house capture ELISA, we aimed to determine if plasma IgM generated in a DENV primary infection could also be detected. Here we showed that IgM in plasma from a child with confirmed primary DENV-2 infection was preferentially serotype-specific, as only DENV-2 but no DENV-1/4 mix-treated wells had higher OD450nm in comparison with mock-treated wells and the negative control (Figure 3). Previous reports have shown that, during primary DENV infections, IgM is only reactive against its corresponding infecting virus(16), but others have described a lack of correlation between IgM responses and the isolated virus serotype, even though the highest levels were found against the infecting serotype(17, 18), then this issue remains to be explored. The use of a previously known cross-reactive mouse anti-DENV antibody which was generated by immunization with DENV-4 (the serotype included in this assay), a dose-response effect after dilution of the sample and acceptable signal / noise ratios support our results. Evidently, more samples have to be analyzed, including a mix with all the DENV serotpyes, to confirm this finding and to corroborate the performance of our ELISA protocol.
In summary, a semiquantitative in-house capture ELISA for the detection of DENV-specific IgM was standardized, and it showed to be effective and comparable with a commercial kit in the measurement of IgM response during primary and secondary infections in children. IgM generated in primary infection was serotype-specific. The use of this assay combined with other direct and indirect methods could be helpful for the diagnosis and studies of virology and immunology of dengue in endemic areas.
To the patients who participated in the study and to the Departmento de Pediatría Hospital Universitario of Neiva. This work was funded by Vicerrectoría de Investigación y Proyección Social of Universidad Surcolombiana.
1. BHATT S, GETHING PW, BRADY OJ, MESSINA JP, FAR-LOW AW, MOYES CL, ET AL. The global distribution and burden of dengue. Nature. 2013;496(7446): 504-7.
2. DENGUE: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition. WHO Guidelines Approved by the Guidelines Review Committee. Geneva2009.
3. SIMMONS CP, FARRAR JJ, NGUYEN V V, WILLS B. Dengue. The New England journal of medicine. 2012;366 (15):1423-32.
4. KORAKA P, SUHARTI C, SETIATI TE, MAIRUHU AT, VAN GORP E, HACK CE, ET AL. Kinetics of dengue virus-specific serum immunoglobulin classes and subclasses correlate with clinical outcome of infection. Journal of clinical microbiology. 2001 ;39( 1 2):4332-8.
5. WAHALA WM, SILVA AM. The human antibody response to dengue virus infection. Viruses. 2011 ;3(1 2):2374-95.
6. VAZQUEZ S, PEREZ AB, RUIZ D, RODRIGUEZ R, PUPO M, CALZADA N, et al. Serological markers during dengue 3 primary and secondary infections. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2005;33(2): 1 32-7.
7. NUNES MR, NUNES NETO JP, CASSEB SM, NUNES KN, MARTINS LC, RODRIGUES SG, ET AL. Evaluation of an immunoglobulin M-specific capture enzyme-linked immunosorbent assay for rapid diagnosis of dengue infection. Journal of virological methods. 2011;! 71 (1 ):1 3-20.
8. GENTRY MK, HENCHAL EA, MCCOWN JM, BRANDT WE, DALRYMPLE JM. Identification of distinct antigenic determinants on dengue-2 virus using monoclonal antibodies. The American journal of tropical medicine and hygiene. 1 982;31 (3 Pt l):548-55.
9. NIGHTINGALE ZD, PATKAR C, ROTHMAN AL. Viral replication and paracrine effects result in distinct, functional responses of dendritic cells following infection with dengue 2 virus. J Leukoc Biol. 2008;84(4):1028-38.
10. PERDOMO-CELIS F, PERILLA P, SALGADO DM, NARVÁEZ CF. Detección y cuantificación de virus dengue 2 en lisado celular y plasma de niños por PCR en tiempo real usando un estuche comercial y el equipo EcoTM System-lllumina. Revista Facultad de Salud. 2014;6(1 ):40-7.
11. LANCIOTTI RS, CALISHER CH, GUBLER DJ, CHANG GJ, VORNDAM AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. Journal of clinical microbiology. 1 992;30(3):545-51.
12. INNIS BL, NISALAK A, NIMMANNITYA S, KUSALERD-CHARIYA S, CHONGSWASDI V, SUNTAYAKORN S, ET AL. An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. The American journal of tropical medicine and hygiene. 1 989;40(4):41 8-27.
13. NARVAEZ CF, FENG N, VASQUEZ C, SEN A, ANGEL J, GREENBERG HB, ET AL. Human rotavirus-specific IgM Memory B cells have differential cloning efficiencies and switch capacities and play a role in antiviral immunity in vivo. Journal of virology. 201 2;86(1 9): 10829-40.
14. CHANAMA S, ANANTAPREECHA S, A AN, SA-GNASANG A, KURANE I, SAWANPANYALERT P. Analysis of specific IgM responses in secondary dengue virus infections: levels and positive rates in comparison with primary infections. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2004;31 (3):1 85-9.
15. CROWTHER JR. The ELISA guidebook. Methods Mol Biol. 2000;149:III-IV, 1-413.
16. BURKE DS. Editor Rapid methods in the laboratory diagnosis of dengue virus infections. International Conference Dengue/Dengue Hemorrhagic Fever; 1983; University of Malaysia, Kuala Lumpur, Malaysia.
17. NAWA M, YAMADA Kl, TAKASAKI T, AKATSUKA T, KURANE I. Serotype-cross-reactive immunoglobulin M responses in dengue virus infections determined by enzyme-linked immunosorbent assay. Clinical and diagnostic laboratory immunology. 2000;7(5):774-7.
18. VAZQUEZ S, LOZANO C, PEREZ AB, CASTELLANOS Y, RUIZ D, CALZADA N, ET AL. Dengue specific immunoglobulins M, A, and E in primary and secondary dengue 4 infected Salvadorian children. Journal of medical virology. 2014;86(9): 1 576-83.